Abstract
Background: ETS Variant Transcription Factor 6 (ETV6, chromosome 12p13), is an essential transcriptional repressor in normal hematopoiesis. Somatic ETV6 mutations are rare but recurrent events in hematologic malignancies, including myeloid neoplasms (MN), and are associated with a negative prognostic impact in MDS (Bernard et al. 2022). We set out to evaluate the prevalence, clinical and co-mutational profile of pathogenic ETV6 mutations in a cohort of patients undergoing targeted next-generation sequencing (NGS) in MNs.
Methods 5,793 consecutive samples from patients with confirmed or suspected MNs submitted for a 42-gene MN NGS clinical assay between 2015 and 2021 at Mayo Clinic Cancer Center (Minnesota, Arizona, Florida) were analyzed for ETV6 mutations (exons 3-8). Mutations deemed pathogenic based on published reports with functional confirmation or presumed to be pathogenic based on in silico analysis were included and referred to as ETV6 'mutated'. Clinical data was recorded by retrospective chart review after IRB approval. BlueSky Statistics was used for statistical analysis.
Results 1) Characteristics: 32 distinct ETV6 mutations were identified across 33 patients with MNs. 3 mutations occurred twice, while 2 patients had 2 ETV6 mutations. At the time of NGS, 23 (70%) had a prior MN diagnosis while 10 (30%) were samples taken at the time of MN diagnosis.
Median hemoglobin was 8.5 g/dL, platelet count 83 x 109/L and white blood cell count 4.4 x 109/L. The median bone marrow blast count, excluding AML or blast transformation (BT), was 4% (n=27). Median age was 71 years (range 40-86), with a male predominance (n=25, 76%). 20 (61%) patients had an abnormal karyotype, with 4 having complex karyotype, 3 having monosomal karyotype, and 1 with concurrent ETV6 mutation and deletion 12p (multi-hit). MDS was the most common diagnosis (n=16, 48%), followed by primary myelofibrosis (PMF) (n=5, 15%), AML with myelodysplasia related changes (n=3, 9%), chronic myelomonocytic leukemia (CMML) (n=3, 9%), CMML- BT(n=2, 6%), AML (n=2, 6%), and single cases classified as MDS/MPN overlap syndrome (3%), and mixed phenotype acute leukemia (3%). 5 MDS cases were classified as MDS-MLD (29%), 5 as MDS-EB1 (29%), 4 as MDS-EB2 (24%), 2 as MDS-U (12%) and a single case (3%) as MDS-RS. The median IPSS-R score was 4, with IPSS-R prognostic groups of: very low (n=2), low (n=3), intermediate (n=3), high (n=3) and very high (n=5). Despite the prevalence of co-mutations and high-risk clinical features, ETV6 mutations were sufficient to move 10/16 patients (63%) to higher risk categories using IPSS-Molecular.
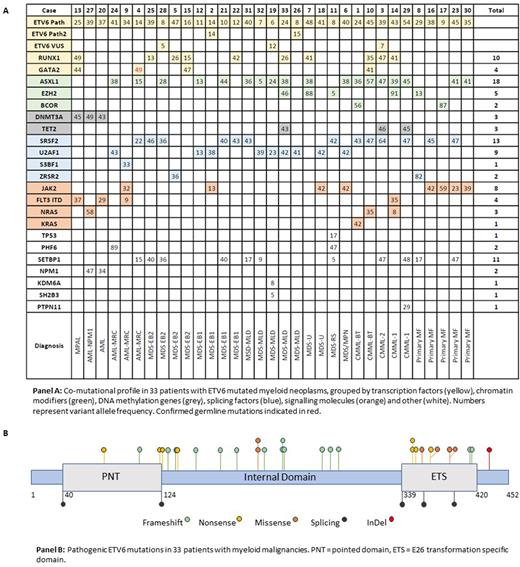
2) Molecular: Co-mutations were always present (median 3.5, range 2-6), and in 3 patients (9%) ETV6 was the dominant mutation measured by variant allele frequency (VAF) (panel A). Median VAF for ETV6 mutations was 35% (range 6-54%). 25 (76%) patients also had splicing factor mutations, 21 (63%) had chromatin modification gene mutations, 14 (42%) had mutations in signaling pathways, and 12 (36%) had mutations in additional transcription factors. ASXL1 (n=18, 55%), SRSF2 (n=13, 39%), and SETBP1 (n=11, 33%) were the most common co-mutations. Frameshift mutations (n=14) were the most common ETV6 mutation subtype and clustered in the internal domain of ETV6 (panel B). Nonsense mutations (n=7) occurred in all 3 domains, while missense mutations (n=5) were primarily found in the ETS domain. 5 mutations involved splicing sites.
3) Survival outcome: Over a median follow up of 25.2 months, the median survival was 17.5 months. One case of leukemic transformation occurred in the MDS cohort over a median 14.1 month follow up, and 5 (31%) within this group proceeded to bone marrow transplant (BMT). Median survival of the AML subgroup was 18.3 months, while that of the MDS-EB cohort was 8.6 months (p=0.33). Median survival for patients undergoing BMT was 18.3 versus 10.5 months for those not (p=0.38). No significant association was found between overall survival and sex, BM blast count, ETV6 VAF, co-mutation number, presence of ASXL1 mutation or abnormal karyotype.
Conclusions In this single-institution cohort, ETV6 mutations occurred rarely in patients being evaluated for MNs, and when detected occurred predominantly in MDS, PMF and CMML. ETV6 mutations were never an isolated abnormality, and rarely represented the dominant clone. ETV6 mutations reclassified the majority of the MDS cohort to a higher prognostic risk category using IPSS-M.
Disclosures
He:Kura Oncology, Inc: Consultancy. Mangaonkar:Bristol Myers Squibb: Research Funding. Patnaik:Kura Oncology, Stem Line Pharmaceuticals: Research Funding. Foran:Novartis, Servier, Pfizer, BMS, Taiho: Other: Formal Advisory Activities; AbbVie, Actinium, Aptose, Astex, H3Biosciences, Kura Oncology, Trillium, Xencor: Research Funding. Shah:Astellas: Research Funding; Celgene: Research Funding; Marker Therapeutics: Research Funding. Al-Kali:Astex: Other: research support to institution.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal